Save articles for later
Add articles to your saved list and come back to them any time.
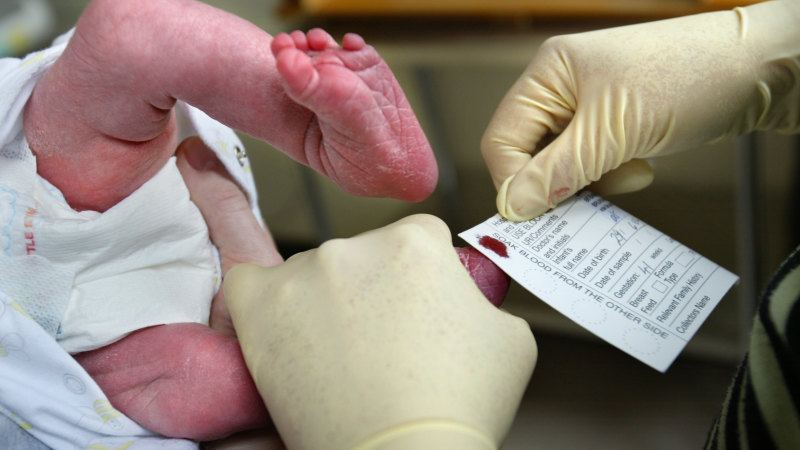
Hours after a baby comes into this world, a moment of pain is inflicted. The newborn squirms and grimaces over a pinprick to the heel; three drops of blood fall onto a paper card, which is whisked away by a nurse.
As the family swaddle their infant, the blood is searched for the signature of a few dozen destructive conditions. Nearly always, nothing is found, and the test is forgotten in the hurly-burly of newborn life.
We could know so much more, if we are willing to ask.Credit: Angela Wylie
Those droplets could show so much more. The technology now exists to extract and unravel a newborn’s chromosomes, revealing their genetic profile. Tests could search for thousands of diseases – even build risk profiles for breast cancer or Alzheimer’s. And cost might not be an issue for long.
Five major studies running across Australia are testing out applying genomic screening to thousands of newborns.
If they can find a way of rolling screening out to the wider public in a reliable and cost-effective way, the question will become: how much do we want to know?
Kirsten Boggs is a genetic counsellor at Sydney Children’s Hospital, Randwick.Credit: Wolter Peeters
“We can’t tell you what we’re going to find,” says Kirsten Boggs, a senior genetic counsellor at Australian Genomics, based at Sydney Children’s Hospital. “Because we don’t know.”
Boggs is 6½ months pregnant. Her child will enter a healthcare system on the cusp of being transformed by genomics.
Professor David Amor, a former director of Victorian Clinical Genetics Services who is now based at the Murdoch Children’s Research Institute, says widely accessible genomic screening is probably inevitable.
“This will be genomics not just for newborn screening, but to the point where everyone in the population will have the option,” he says.
Just two years ago, Amor thought routine genomic newborn screening was a scientist’s pipedream. No longer. “I’ve come around,” he says. “We’re preparing for the world of the future.”
But newborn genomic screening has a particular set of societal challenges.
Current screening is deliberately restrictive; tests look for just a few dozen diseases, such as cystic fibrosis. They could look for more – type 1 diabetes risk, for example – but don’t. Society has decided childrenshould have the right to grow for a while at least blissfully unaware of any future peril.
Genomic screening will allow parents and doctors to know so much more – if they are willing to ask. Science has already picked out more than 4000 genes known to cause disease; that number will only grow. Because there are so many genes, looking too closely will more than likely reveal something wrong.
“You don’t do a genome of 22,000 genes and not find anything,” says Professor Chris Semsarian, who researches genetic heart disease at the University of Sydney. “If I sequence your genome today, I guarantee you’ll become a patient.”
A trial of newborn genomic screening in the US spotted single-gene disease risks in 11 per cent of newborns; 5 per cent had a gene that could influence how their body responds to certain mutations. Some 88 per cent of newborns had a harmful recessive gene that could be passed on to their children.
Genome sequencing can spot problems with no cures.
Boggs says: “Potentially we may find something that’s not treatable. Are you ready to hear that information? I guess no one can really be ready. Once you start down that trajectory, once you know, you can’t un-know.”
Or sequencing can provide uncertainty. For reasons not fully understood, some people can have a disease-causing gene but no disease. Geneticists call this “penetrance”.
Says Amor: “It’s tempting to think genetics tells us we’ll get a disease or not. But genetics is not absolute.”
Open the genetic box and all sorts of things could come out. That’s why “we’re going to operate in a very conservative approach”, says clinical professor Bruce Bennetts, who leads one of the five screening studies.
If genomic screening is rolled out, it’s likely that tests will only look for genes that cause diseases that can be treated immediately, just as current screening does.
Boggs says there should be more genetic counsellors and that midwives, doctors and paediatricians should be trained in this area. The conversations are difficult, the ethics are tricky, and the work is often harrowing.
One family Boggs worked with had pre-birth screening – known as noninvasive prenatal testing – which came back clear. These tests look for common genetic conditions, such as Down syndrome, but they cannot spot every disease, and this family’s child was born critically ill. A more comprehensive genetic test revealed a rare condition that would have led to profound physical and intellectual disability.
Surgeons wanted to operate on the child and were pressuring the family. But counsellors must also be advocates, Boggs says. The parents asked her: “Do we have a say?”
Boggs told them it was their choice. They opted against the surgery; the child passed away at home, surrounded by family.
In other cases, families of sick children must mull over having a genetic test at all. The results may indicate an incurable illness.
“Sometimes people don’t want that hope taken away, so they will decline or delay testing,” Boggs says.
But hope can spring from the tests too. Natasha Wagner’s daughter, Evie, woke up one day entirely yellow. Her liver was failing. She was rushed to hospital. Blood tests revealed internal chaos. Everything was wrong, but for a week, no one could say what was wrong exactly.
A genetic test eventually revealed Wilson’s disease, in which the liver and brain accumulate copper. Evie needed a new liver. With treatment, she is now thriving.
After counselling, Wagner opted to have her other six children genetically tested. “[The counselling] was lovely. It’s like, why would you want to test them, it’s really rare? But we had a child who had it, and three others who might,” she says.
The benefits of newborn genetic testing are most apparent for children like Evie: it could have caught the disease immediately. “If you could screen for them, it may help being proactive,” Wagner says.
Liam Mannix’s Examine newsletter explains and analyses science with a rigorous focus on the evidence. Sign up to get it each week.
Most Viewed in National
From our partners
Source: Read Full Article